 Mohammad Parhizi, a post-doctoral researcher with the Purdue Polytechnic Institute, has long been interested in heat transfer – a good fit for the work done in the college’s Applied Thermofluids Laboratory and Fuel Laboratory of Renewable Energy. About a year ago, working with Jason Ostanek, assistant professor of engineering technology, and Gozdem Kilaz, associate professor of engineering technology, Parhizi began his current research, “The chemical and thermophysical characterization of electrolytes within lithium ion batteries.”
Mohammad Parhizi, a post-doctoral researcher with the Purdue Polytechnic Institute, has long been interested in heat transfer – a good fit for the work done in the college’s Applied Thermofluids Laboratory and Fuel Laboratory of Renewable Energy. About a year ago, working with Jason Ostanek, assistant professor of engineering technology, and Gozdem Kilaz, associate professor of engineering technology, Parhizi began his current research, “The chemical and thermophysical characterization of electrolytes within lithium ion batteries.”
Simply put, Parhizi’s work examines heat transfer in the inner workings of lithium ion (“Li-ion”) batteries, which have high power density and can provide energy in an efficient way, in order to identify safety parameters and limitations related to the composition of Li-ion components.
“We know that the world is transitioning from fossil fuels to renewable energy, and Li-ion batteries are an important part of that transition,” said Parhizi. “These batteries are in our consumer electronics, power grids, vehicles, and even military and space applications.
“But, under certain conditions, Li-ion batteries can generate a huge amount of heat, which creates a safety concern. We are using modeling and experiments to study the behavior of the batteries in abuse conditions.”
Creating a new research methodology
The first step of this research involved careful examination of the contents of Li-ion batteries. The makeup of the electrolyte within a Li-ion battery solution – the unique, proprietary fluid that makes it possible for the battery to function – varies from one manufacturer to another, and the precise makeup of the electrolyte is not shared publicly. This defined the first phase of Parhizi’s research: developing a precise methodology to investigate the Li-ion electrolyte components that play a key role in the potential severity of thermal transfer issues.
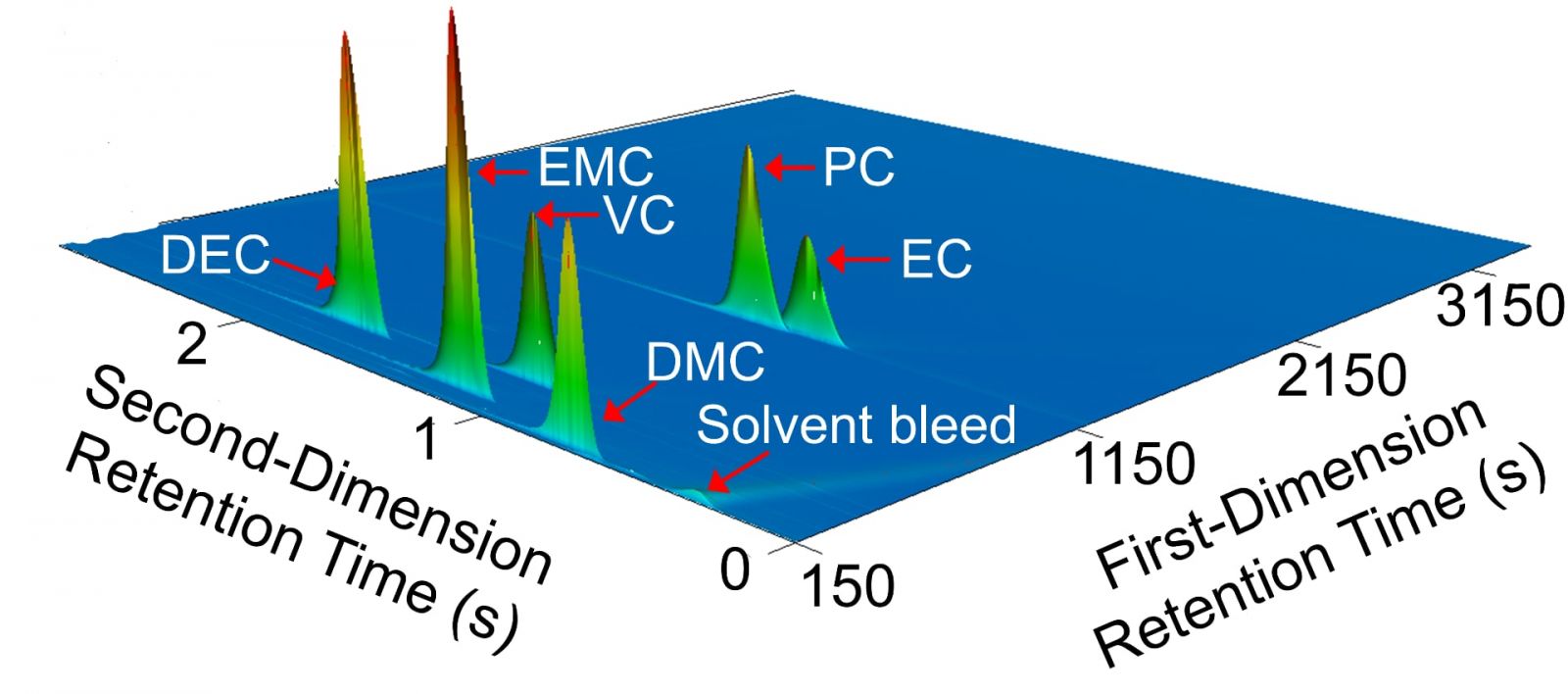
To develop his methodology, Parhizi collaborated with the Polytechnic Institute and Purdue’s Department of Chemistry for knowledge, training and hands-on work — and faced the additional challenge of explaining his work to people in other departments with differing areas of expertise. Along the way, he picked up a few new skills that will aid in this and future research.
“This is my first experience in conducting highly interdisciplinary research,” said Parhizi. “Most of my previous work focused on theoretical modeling of heat and mass transfer, while this work is based on analytical methods in chemistry. So, I audited a chemistry class, learned how to use state-of-the-art instruments, and learned how to interpret the data. It has been challenging and interesting.”
Looking to the future
For the first year of the project, Parhizi and his team created their own electrolyte solutions to develop and prove a reliable methodology for precise identification of electrolyte components in Li-ion batteries. Next, he plans to model abuse conditions, such as placing the batteries in ovens, to determine how specific electrolyte solutions perform under stress.
“For this new testing methodology, our goal is to publish our methodology and make it available to the companies and other organizations that need to evaluate the Li-ion batteries they purchase and use.”
Parhizi’s hope is that industry, government and military organizations will use his research to better understand the range of safety for the batteries they use.
“Ultimately,” said Parhizi, “the intent is to identify points of failure and fully understand the conditions under which Li-ion batteries are safe.”
